

The number of neutrons is therefore the difference between the mass number and the atomic number: A – Z = number of neutrons. The total number of protons and neutrons in an atom is called its mass number (A). Therefore, the atomic number also indicates the number of electrons in an atom.
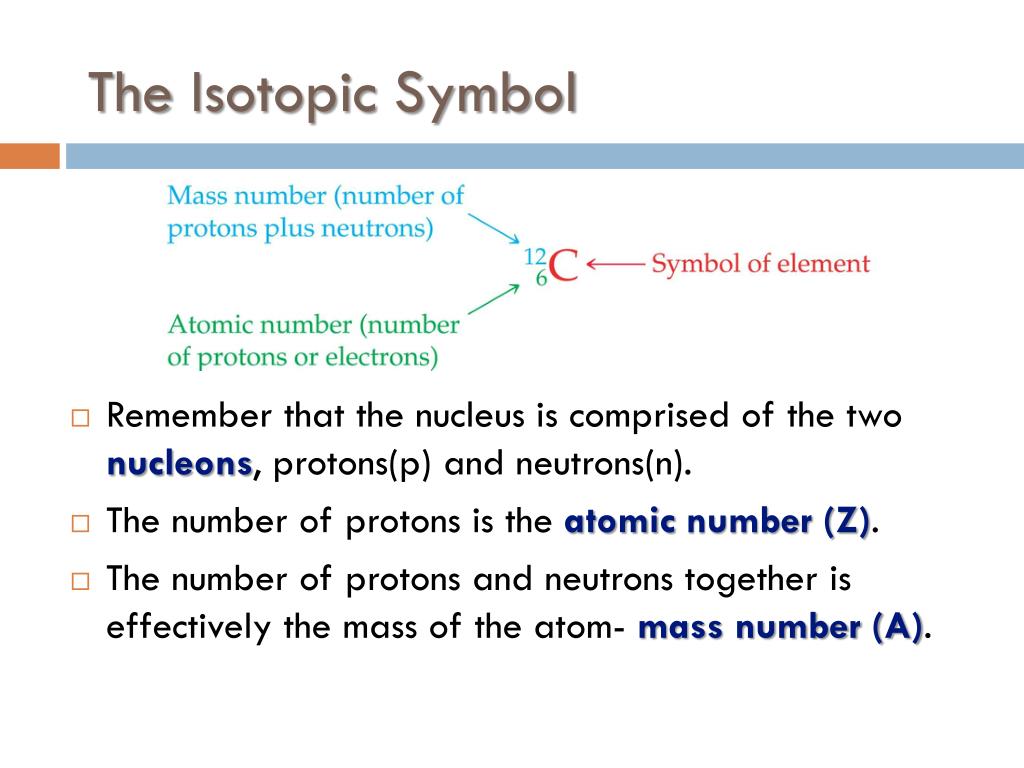
A neutral atom must contain the same number of positive and negative charges, so the number of protons equals the number of electrons. This is the defining trait of an element: Its value determines the identity of the atom. The number of protons in the nucleus of an atom is its atomic number (Z). The electron has a charge of 1− and is a much lighter particle with a mass of about 0.00055 amu (it would take about 1800 electrons to equal the mass of one proton. A neutron is a slightly heavier particle with a mass 1.0087 amu and a charge of zero as its name suggests, it is neutral. The fundamental unit of charge (also called the elementary charge) equals the magnitude of the charge of an electron (e) with e = 1.602 × 10 −19 C.Ī proton has a mass of 1.0073 amu and a charge of 1+. The amu was originally defined based on hydrogen, the lightest element, but now is defined with regard to the most abundant isotope of carbon, atoms of which are assigned masses of exactly 12 amu. When describing the properties of tiny objects such as atoms, we use appropriately small units of measure, such as the atomic mass unit (amu) and the fundamental unit of charge (e). For example, a carbon atom weighs less than 2 × 10 −23 g, and an electron has a charge of less than 2 × 10 −19 C (coulomb). (credit middle: modification of work by “babyknight”/Wikimedia Commons credit right: modification of work by Paxson Woelber)Ītoms-and the protons, neutrons, and electrons that compose them-are extremely small. If an atom could be expanded to the size of a football stadium, the nucleus would be the size of a single blueberry. For a perspective about their relative sizes, consider this: If the nucleus were the size of a blueberry, the atom would be about the size of a football stadium ( Figure 1)! Figure 1. The diameter of an atom is on the order of 10 −10 m, whereas the diameter of the nucleus is roughly 10 −15 m-about 100,000 times smaller. The nucleus contains the majority of an atom’s mass because protons and neutrons are much heavier than electrons, whereas electrons occupy almost all of an atom’s volume. It was learned that an atom contains a very small nucleus composed of positively charged protons and uncharged neutrons, surrounded by a much larger volume of space containing negatively charged electrons. The development of modern atomic theory revealed much about the inner structure of atoms.

Define the atomic mass unit and average atomic mass.Write and interpret symbols that depict the atomic number, mass number, and charge of an atom or ion.By the end of this section, you will be able to:


 0 kommentar(er)
0 kommentar(er)
